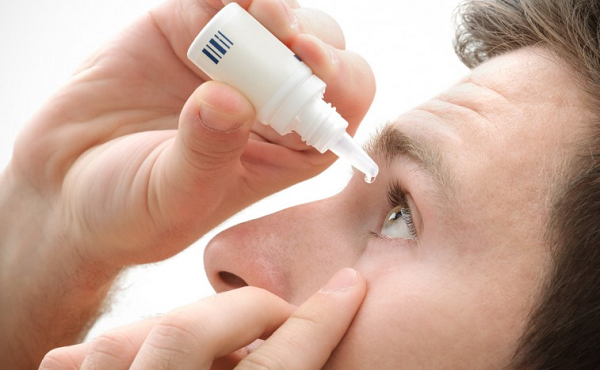
Shire has won United States endorsement for its the most imperative new medication, the treatment for dry eyes, in a new help for the organization which finished its Thirty Two USD billion procurement of United States uncommon ailments pro Baxalta a month ago.
The Dublin based drugmaker has always been a major securities exchange champ taking after England’s choice to abscond the European Union, profiting from the quality of the U.S. dollar against sterling and speculator interest for protective divisions like pharmaceuticals.
Shire offers – as of now up a fifth since the UK submission on 23rd June, increased another 4% on Tuesday on news the United State Sustenance and Medication Organization had endorsed lifitegrast eye drops for treating side effects and signs of dry eye infection.
Lifitegrast, will be promoted as Xiidra in the Assembled States, is relied upon to dispatch in the second from last quarter. It treats an eye illness that influences almost 16 million grown-ups in the Unified States.
It is Shire’s first FDA-affirmed pharmaceutical in ophthalmics and imprints an imperative initial phase in the gathering’s desire to wind up a pioneer in this business sector.
More
It is likewise the primary new medication to treat the condition following Allergan’s (AGN.N) Restasis won a green light in 2002 and examiners said the mark depiction for Shire’s item contrasted positively and its rival.
Agreement conjectures at present point to yearly Xiidra offers of a little more than a $1 billion in 2021, $1.4 billion expected for Restasis this year as per Thomson Reuters Cortellis. Yet, that Shire figure may now be reconsidered up.
“With a solid name and better bearableness profile, Xiidra could improve,” said by Berenberg Bank examiner Alistair Campbell.
Experts of Industry accept there is still a huge open door in the dry eye market, as present medications, for example, steroids and Restasis are a long way from ideal.
Xiidra had clear advantages over old items, since it acts as ahead of schedule as 2 weeks after treatment, when Restasis can take 6 to 8 weeks, as indicated by ophthalmology authorities, Leerink investigator Jason Gerberry said.
Xiidra is additionally shown for a treatment of both side effects and signs of dry eyes infection, while Restasis was endorsed just on an exhibited change in target indications of dry eyes.
A frequently unending illness, dry eye is connected with aggravation that may in the long run lead to harm to the surface of the eye.
Shire’s medication had been dismisses at first by the Food Department Authorities in October, then Shire was requested an extra clinical concentrate, yet the organization had stayed certain that it could fulfil the U.S. controller.