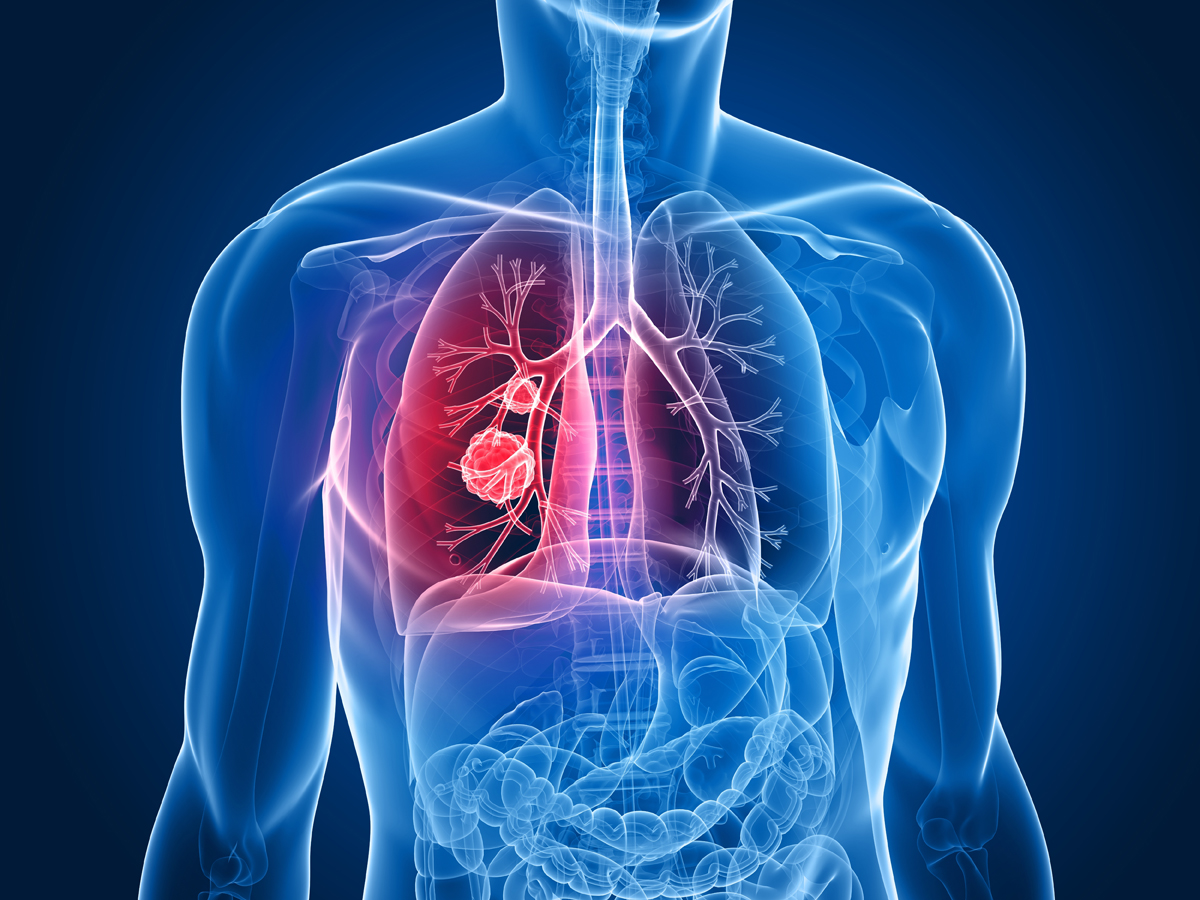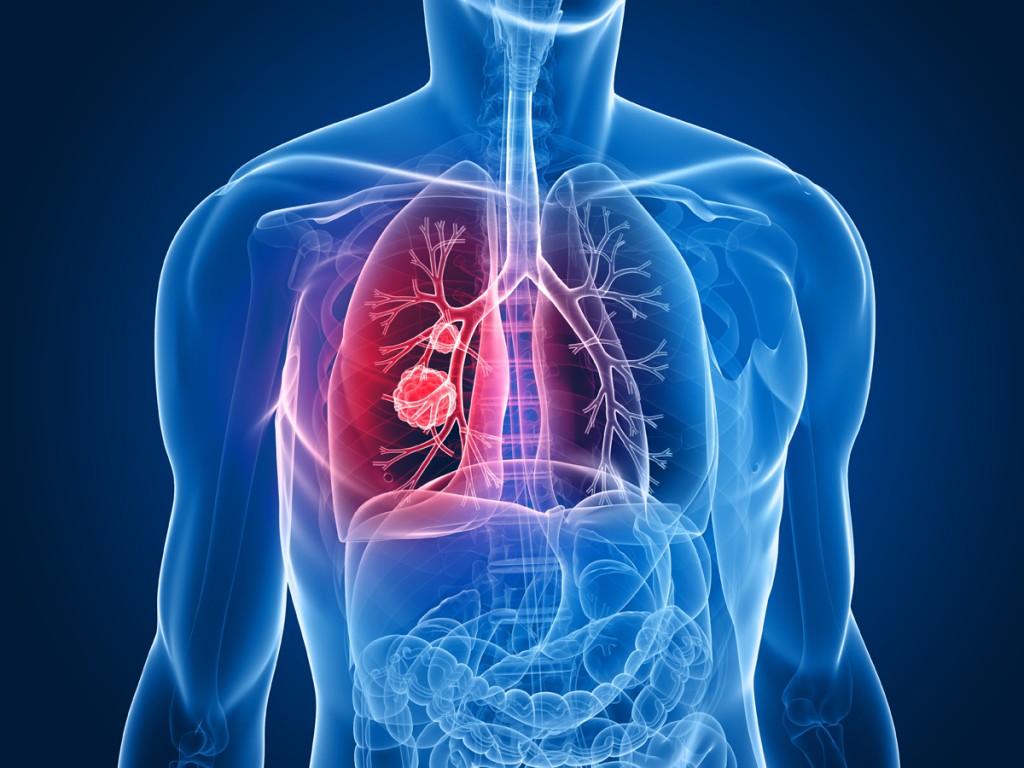
But Keytruda offers new hope for lung cancer patients.
Keytruda (pembrolizumab) is a new immune therapy drug from Merck. It belongs to a new category of drugs which “simulates the body’s immune system,” as described by Merck CEO Kenneth Frazier. In an interview with Fox Business news, Frazier goes on to explain “With respect to people who are the sickest lung cancer patients, we now know, using Keytruda will extend their life by approximately 13 months on average. We know that it will reduce the risk of death by 30-40% for people who had failed on standard chemotherapy.”
For the most recent study, researchers compared the drug with the chemotherapy drug docetaxel among 1,000 patients who have been diagnosed with non-small cell lung cancer. This is the most common form of the disease.
All of the patients involved with the study had persistent tumors which had progressed, even after completing rounds of chemotherapy. The tumors of these patients had all produced a protein called PD-L1. This protein can protect the tumor from attacks by the immune system.
Study leader Dr. Roy Herbst is a professor of medicine with Yale University School of Medicine. He notes that the findings suggest this drug has the potential to be offered to patients at an earlier stage of disease progression, those who are found to have a specific lung tumor profile.
Also the chief of medical oncology at the Yale Cancer Center and Smilow Cancer Hospital, Dr. Herbst continues, “I believe we should treat patients with the best available drugs as soon as possible. Now that we have learned which patients are most likely to benefit from the anti-PD-L1 strategy, we could begin moving this drug to the earlier setting stages,” he said in a Yale news release.